Gavi, the Vaccine Alliance continues to be deeply concerned about the escalating mpox emergency on the African continent. We welcome recently announced measures by our partners at the African Centres for Disease Control and Prevention (Africa CDC) and World Health Organisation (WHO) which will help create an enabling environment in support of a comprehensive region-led response, and the join partners in emphasizing the importance of international coordination following a declaration of a Public Health Emergency of Continental Security by Africa CDC and a Public Health Emergency of International Concern by WHO.
Working in collaboration with countries and partners, Gavi has been closely monitoring the mpox situation since 2022. As a result, considerations related to mpox have helped inform both the design of Gavi’s new innovative health security mechanisms, aimed at addressing systemic gaps exposed during the COVID-19 vaccine response, as well as Gavi’s next five year strategy. When the assessment process for Gavi’s next five-year Vaccine Investment Strategy (VIS) began in 2023, mpox vaccines were included for consideration. Special sessions of the VIS Steering Committee were convened in Q1 2024 to ensure the approach took into account developments in the Democratic Republic of the Congo (DRC), where the outbreak has spread rapidly, primarily impacting children (in terms of both morbidity and mortality), and resulting in a high case fatality ratio.
As a result, in June 2024 the Gavi Board approved the following measures related to mpox:
- In its next five-year strategic period, beginning 2026, Gavi will establish a global stockpile of mpox vaccines – similar to its existing stockpiles for cholera, Ebola, meningitis and yellow fever vaccines. Establishment of a stockpile is pending the availability of WHO Emergency Use Listing or prequalification of a recommended vaccine, and subject to fundraising for Gavi’s next strategic period. A critical goal will be ensuring the design of the stockpile is informed by a robust assessment of the long-term public health need.
- In the interim, Gavi will support outbreak response in the DRC and surrounding countries. More details on this are available below.
- In parallel, Gavi will make critical investments in a learning agenda that will ensure the current response will help inform and improve future vaccination efforts against the disease, including the design of a global stockpile. More details on this are available below.
Support to the on-going emergency and response efforts
Gavi has been monitoring the mpox situation daily for the past several weeks, and is currently engaging closely with countries, Africa CDC, WHO, UNICEF, donors, and manufacturers to support the response to mpox. Our actions include:
- Rapid operational support: As an immediate measure, Gavi has formally declared the mpox situation a regional emergency. This allows us to trigger certain additional flexibilities and streamline processes, underpinned by a no-regrets approach to risk. Specifically, this could enable repurposing available funds in support of vaccine response (for example, funding for operational costs to support use of dose donations) and waiving of the formal independent review process for new vaccine introductions and campaigns.
- Leveraging new innovative mechanisms to support the overall response: In June 2024, the Gavi Board also approved the final terms of the First Response Fund, the fastest tool in a suite of instruments called the Day Zero Financing Facility, which – in response to a key learning from COVID-19 – seeks to make resources immediately available for a vaccine response to an urgent public health emergency. Gavi could draw on the First Response Fund, which can be accessed in the event of a WHO declaration of a Grade 2 or Grade 3 emergency or PHEIC, to support both direct procurement of mpox vaccines as well as country readiness and other aspects of the vaccine response.
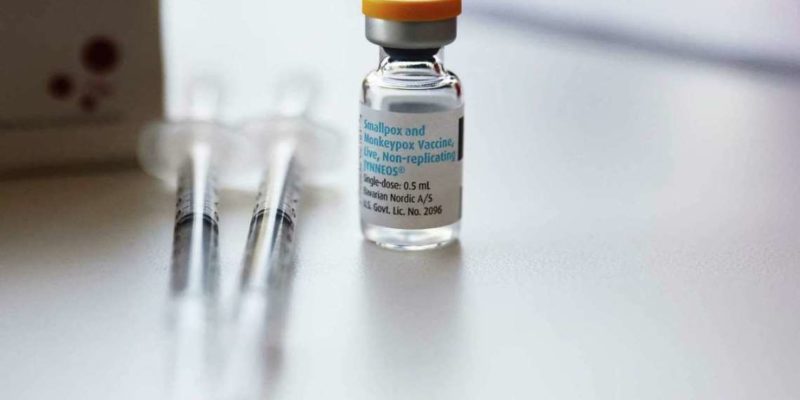
- Direct procurement: Gavi has accelerated engagement with manufacturers, including Bavarian Nordic, for potential direct procurement of mpox vaccines in support of outbreak response. WHO has recently announced that two vaccines are under consideration for Emergency Use Listing, and this is an important interim measure enabling partners to act now, in advance of the longer timeframe expected for WHO prequalification.
- Dose donations and emergency response: Building on the unique experience of working with 33 donors to coordinate the supply of nearly 1 billion dose donations to 114 countries in response to COVID-19, Gavi will offer direct support and share knowledge with countries and partners as requested, while working with them on designing in-country vaccination strategies. In a workshop last month, the Government of DRC, Africa CDC, Gavi, WHO, UNICEF, and other partners worked together to design a three-phase vaccine response strategy, informed by a collective understanding of the available supply of dose donations. In parallel, Gavi is offering to share legal and process knowledge and provide operational support to Africa CDC and other partners who are also engaged in sourcing dose donations. Based on our experience working with donors, countries and partners to coordinate the global COVAX dose donation mechanism, this includes providing information on the complex technical, legal, regulatory and logistical considerations involved in mounting rapid vaccination campaigns with donated doses.
- Investing in a learning agenda: Following the June 2024 Board decision, Gavi has moved to ensure any vaccine response for mpox will also help improve the data available on disease burden and epidemiology, including through supporting surveillance studies and modelling studies on vaccine use and impact. Expanding the available data will support the work of countries and partners such as WHO and Africa CDC, and ensure a future global stockpile accurately addresses the longer-term public health need – thus helping improve future vaccination efforts against the disease. Following recent developments, Gavi is in the process of working with partners to finalize the design of this agenda, with the aim issue a Request for Proposals as soon as possible.